
Of course, just like your annual check-up with your physician, the more tests the better. But just like your physician, nothing is free. So what can you test for on-site to save some dollars while being able to monitor your water quality in the greenhouse?
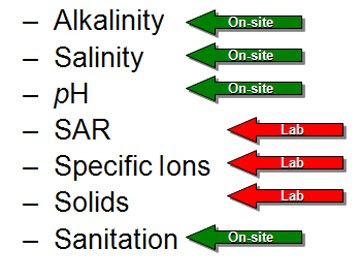
With the correct instruments and test kits you can test for many conditions on-site, including Alkalinity, salinity, pH, and sanitation. The next few blog postings, I will be covering techniques that you can use at your own greenhouse operation. As always, these on-site tests do not replace laboratory testing, but are a supplement. For determining the sodium absorption ratio (SAR), specific ion concentrations and solids, you still must use a laboratory.
 One of the first things that a greenhouse grower needs to know about their water is the alkalinity. Alkalinity is defined as the capacity of water to neutralize acids. Anions typically found in water are bicarbonate (HCO3-) from dissolved salts such as calcium bicarbonate (Ca(HCO3)2), sodium bicarbonate (NaHCO3), and magnesium bicarbonate Mg(HCO3)2); and carbonate (CO3--) from dissolved salts such as calcium carbonate (CaCO3).
One of the first things that a greenhouse grower needs to know about their water is the alkalinity. Alkalinity is defined as the capacity of water to neutralize acids. Anions typically found in water are bicarbonate (HCO3-) from dissolved salts such as calcium bicarbonate (Ca(HCO3)2), sodium bicarbonate (NaHCO3), and magnesium bicarbonate Mg(HCO3)2); and carbonate (CO3--) from dissolved salts such as calcium carbonate (CaCO3).
Alkalinity is not be confused with the term alkaline, which is where the pH is greater than 7. Laboratories often report alkalinity as a calcium carbonate equivalent, using milligrams per liter (ppm) of calcium carbonate. Some laboratories will report carbonates in molar values (meq/L). The key to alkalinity issues is information and how it will impact your greenhouse production. For most greenhouse crops, an alkalinity from 40-120 ppm CaCO3 is safe. About 40% of U.S. irrigation water is greater than 200 ppm and 18% of U.S. irrigation water is less than 40 ppm. when the alkalinity of your irrigation water is high, it may raise the pH of your soil medium over time making some nutrients less available. To compound this issue, the smaller the soil medium volume, the more rapid the change. Therefore, plug production requires water with low alkalinity. The key is to adapt your nutrient management practices to your water source.
The key to alkalinity issues is information and how it will impact your greenhouse production. For most greenhouse crops, an alkalinity from 40-120 ppm CaCO3 is safe. About 40% of U.S. irrigation water is greater than 200 ppm and 18% of U.S. irrigation water is less than 40 ppm. when the alkalinity of your irrigation water is high, it may raise the pH of your soil medium over time making some nutrients less available. To compound this issue, the smaller the soil medium volume, the more rapid the change. Therefore, plug production requires water with low alkalinity. The key is to adapt your nutrient management practices to your water source.
Acid injection is a convenient means to neutralize alkalinity in irrigation water. Sulfuric acid is the least expensive followed by nitric, phosphoric, and citric.
 Most growers use sulfuric acid, but some use nitric, which may fume. Phosphoric is often expensive and may disrupt the phosphate balance of your fertility program. Citric acid is a possibility when an inexpensive source is available.
Most growers use sulfuric acid, but some use nitric, which may fume. Phosphoric is often expensive and may disrupt the phosphate balance of your fertility program. Citric acid is a possibility when an inexpensive source is available.
The next question to ask is how much acid should be injected to neutralize the alkalinity? Before that question is asked, first you must determine the alkalinity in your water. Of course, the best means is to send your water to a laboratory for testing, but there are inexpensive test kits that are quite good. The kit below is manufactured by HACH Company of Loveland, Colo. HACH manufactures analytical instruments and reagents used to test water quality1. Many of them are inexpensive and very easy to use. This Alkalinity Test Kit is currently priced at $37.89 for approximately 100 tests. That is less than 40 cents per test and you can not buy a cup of coffee for that. It is accurate within 5 ppm.
 This test kit identifies the presence of alkalinity in irrigation water through titration with sulfuric acid. The colorimetric reagents are phenolphthalein and bromocresol green-methyl red. It is actually a fun test to conduct with kids as the color changes with pH.
This test kit identifies the presence of alkalinity in irrigation water through titration with sulfuric acid. The colorimetric reagents are phenolphthalein and bromocresol green-methyl red. It is actually a fun test to conduct with kids as the color changes with pH.

An excellent resource for greenhouse growers for managing alkalinity was written by Dr. Doug Bailey while he was at North Carolina State University - ALKALINITY CONTROL FOR IRRIGATION WATER USED IN GREENHOUSES. This publication is easy to understand with specific recommendations. There are many more publications available on the NCSU Floriculture Science web site.
1The information contained herein is provided as a public service with the understanding that Colorado State University makes no warranties, either expressed or implied, concerning the accuracy, completeness, reliability, or suitability of the information. Nor does Colorado State University warrant that the use of this information is free of any claims of copyright infringement. Colorado State University do not endorse any commercial providers or their products.
1 comment:
I wanted to know recommended values of PH, tem, alk in water , thanks for your post with sample A and sample B it cleared it out.
Post a Comment